
|
 Chemistry - Metals 3 Chemistry - Metals 3

Under the microscope, the lattice structure can be seen after the surface has been treated accordingly. Not only simple atoms or ions can form crystal lattices, but also molecules and their groups. Also the crystal
formation is not only limited to metals, as already the example of ice crystals shows. The term 'crystalline' only refers to an order in the structure. This can refer to subareas or an overall structure.

'Crystallisation' means that atoms arrange themselves in a certain form under certain conditions. In the example above, one atom is arranged on each corner of a cube, but is of no particular importance for the metals we
are interested in for this chapter. These include, for example, tungsten (W), chromium (Cr), tantalum (Ta), niobium (Nb) and molybdenum (Mo), which are arranged as shown in the picture below. The packing densities
are 68 percent at the top and 74 percent at the bottom.
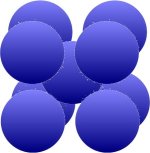
| Additional atom at the intersection of the space diagonal |
The grid is also called cubic-space-centered, which is easier to understand when compared to the cubic surface-centered grid at the bottom. This can be found in copper (Cu), nickel (Ni), aluminum (Al), silver (Ag) and
gold (Au). First result: These two arrangements allow a much more compact package than the first one above.
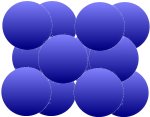
| Additional atoms in the intersections of the surface diagonals |
The next structure allows the same packing density of 74 percent. This time it is achieved by a hexagonal arrangement around an atom. In between there are one (picture below) or two (picture below) groups of three,
which also move close to each other and fill at least three of the six gaps.
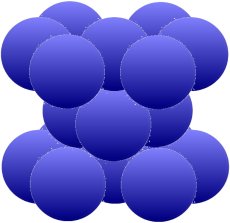
The arrangement above is typical for magnesium (Mg), zinc (Zn), zircon (Zr) and cadmium (Cd). The iron (Fe) and also titanium (Ti), cobalt (Co), tin (Sn) and manganese (Mn) which are so important for us can form
different lattice structures depending on the temperature. Below, two groups of three are grouped, twisted by 120° against each other, between the hexagonal rings.
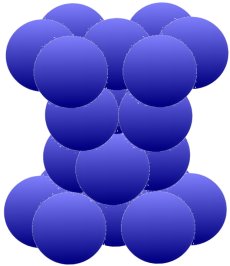
Metals arrange themselves in these lattice structures when, for example, they change from a liquid to a solid state when they cool down. Liquids are basically disordered. But since there is always different crystallization
nuclei at the onset of strength, the structures formed in this way rarely fit together. Errors in the lattice locations are always found in so-called 'real structures'.
However, errors are also possible within a single lattice structure, e.g. when a lattice space is not occupied. The higher the temperature, the more frequent such errors are. Technically, lattice defects can cause a
improvement, but also harm with regard to the required properties. That is why there is a desire to influence properties. This is called heat treatment and not only cooling, but can also include an increase in temperature
in between, e.g. during hardening and tempering.
|
|