 Chemie - Quantum Mechanics 2 Chemie - Quantum Mechanics 2
As already said, no experiment has disproved the existence of quantum mechanics, but many can no longer be explained with classical physics. So only with it we
are able to describe the following experiment. Previously we have to explain what a photon is.
According to quantum mechanics, electromagnetic radiation is a kind of transport of so-called 'energy quanta' through space, each of which is indivisible.
Incidentally, Einstein has received the Nobel Prize for this theory, not for the much more familiar theory of relativity.
We therefore produce photons, in this case light quanta, and throw them onto a device which makes the respective impact of a photon visible. Since this is a highly
precise device and its position is not changed, we would expect a constant impact point. You can guess, there is a wide spread like a shotgun.
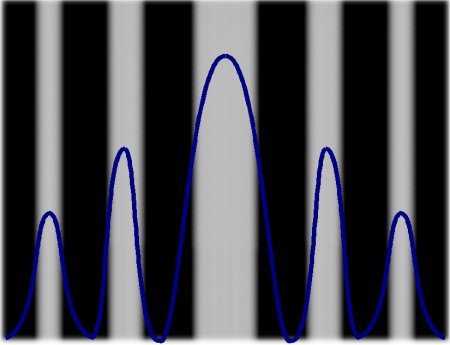
The next experiment is performed with a metal wall between the photon generator and the impact surface, which has two identical perpendicular openings next to
one another of a few millimeters width. The photons have to pass through it, and after the first attempt, two surfaces of photons are expected.
Of course this is not the case, otherwise we would not mention the attempt here. In spite of only two gaps and otherwise unchanged experimental setups, five
distinctly separated fields and, in addition, a different size are formed, decreasing on each side from the center. Such a pattern is only explicable if one assigns a
wave character to the photon.
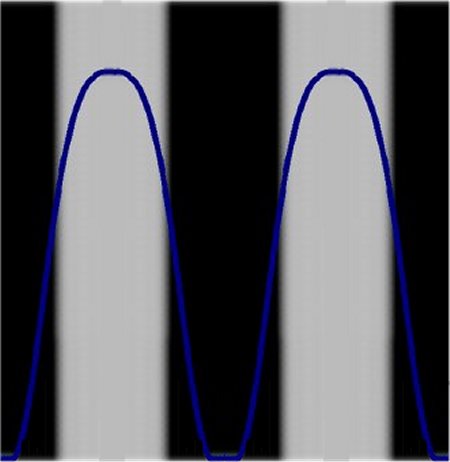
But now it becomes cmore onfusing. If the test is repeated with a measuring device at the gap, then this counts every passage. It seems as if the exact location of a
photon would only be as long as one measures. In order to make the extent of the confusion complete, the two areas that were actually expected before the trial
now appear at the impact surface.
Photons can move through air without great losses. However, if one takes matter of e.g. In the form of electrons or atoms, the same effect can be obtained, but only
in the airless space. As a result, the double gap test shows both the wave as well as the respective particle characteristics. And finally, the location e.g. of an
electron only with a specific probability can be determined.
Although quantum mechanics has much more colliding phenomena in our backpack with our reality, we will try to make a closer explanation for this experiment.
Imagine only the blood pressure measurement on a heart patient. Can it be that the measured value is always too high, because the patient is simply afraid of the
measurement?
There are also measurements in our life that can not be carried out independently of the measurement event. But, of course, this does not explain the completely
different pattern after attaching measuring devices. This is probably why the double-slit experiment is so famous.
|