
|
 Atomic model 1 Atomic model 1
The order of the world has been decoded, at least down to the level of the atoms. The Greeks Leucippus and Democritus coined the term around 500 years before Christ. They assumed that all matter is divisible up to a
certain size and then no more. Well, it is divisible after all, the 'indivisible'. But it is amazing that this construct has lasted so long and will continue to do so.
Significant new discoveries were made here more than a hundred years ago. But you always worked with models. It's only been a good ten years since the
scanning tunneling microscope make atomic structures visible at all. You mainly work with models that are kept just complicated
enough to answer certain questions.
| Proton | Carrier of positive charge, has 1,836 times the mass of an electron, together with the neutrons forms the atomic nucleus. |
| Electron | Carrier of negative charge, balances positive of atomic nucleus, fills a space 100,000 times larger than that of atomic nucleus. |
| Neutron | Electrically neutral, about the same weight as a proton, is found in all atomic nuclei except hydrogen, 1 to 1.5 times as often as protons. |
In order not to stay too long with different atomic models, Bohr's atomic model provides a sufficient basis for the time being. However, it is important to add that a model only makes sense as long as it is relevant to the
explanation of the real relationships. One must not confuse the model with reality.
Why only hydrogen has no neutrons? Probably because it only has one proton. If there were two, they would repel each other because of their positive charges. Neutrons obviously ensure that the atomic nucleus stays
together, but they are constantly moving at around 60,000 km/s.
| Elementary charge | Smallest charge of free particles. Each occurring charge is an integral multiple of the elementary charge. |
| Atomic number | Number of elementary positive charges, corresponds to the number of protons in the atomic nucleus. |
Up to a certain size, one neutron per proton is obviously sufficient for cohesion. With more than 20 protons in the nucleus, the same number of neutrons is obviously no longer sufficient to hold it together. Then it becomes
confusing. Calcium has 20 protons and 20 neutrons each, scandium then with 21 protons already 24 neutrons.
| Element | Protons | Neutrons | Ratio
|
| Calcium | 20 | 20 | 1,00 |
| Scandium | 21 | 24 | 1,14 |
| Titanium | 22 | 26 | 1,18 |
| Vanadium | 23 | 28 | 1,22 |
| Chrome | 24 | 28 | 1,17 |
| Manganese | 25 | 30 | 1,20 |
| Iron | 26 | 30 | 1,20 |
| Cobalt | 27 | 32 | 1,19 |
| Nickel | 28 | 31 | 1,11 |
At some point there can be almost any number of neutrons in the nucleus, cohesion can simply no longer be guaranteed. Below is the last atom with a stable nucleus, bismuth, listed first. What happens if the nucleus is not
stable? It decays until there are at most 84 protons.
| Element | Protons | Neutrons | Ratio
|
| Bismuth | 83 | 126 | 1,51 |
| Polonium | 84 | 126 | 1,50 |
| Astat | 85 | 125 | 1,47 |
| Radon | 86 | 125 | 1,47 |
| Francium | 87 | 126 | 1,45 |
| Radium | 88 | 128 | 1,45 |
| 89-91 | | |
| Uranium | 92 | 146 | 1,59 |
| Neptunium | 93 | 144 | 1,55 |
| Plutonium | 94 | 150 | 1,60 |
Since this process also produces different-sized parts of the nucleus, it is called radioactive decay. For example, there can be alpha rays, which consist of two protons and two neutrons (corresponds to the helium nucleus).
So they are debris parts of the atomic nucleus. Already a sheet of paper can protect you against them.
Beta rays, electrons, also come from the nucleus, which makes the attentive reader sit up and take notice. They probably arise from the high-energy fragmentation of neutrons. Perhaps this also explains why the mass of the
neutron is greater than that of the proton by that of an electron. Here, a somewhat longer distance through the air is enough to protect us from this radiation.
To make the first confusion with electrons from smashed neutrons complete, it should be mentioned that then so-called positrons are also created, i.e. positively charged electrons. They are to be assigned to the so-called
antimatter, are even less effective and should be put aside for our further considerations.
That leaves the gamma radiation, which is fundamentally different from the previous two. It is extremely short-wave light, much more penetrating than X-rays, for example. So it's no matter, which significantly increases its
dangerousness. Probably only thick lead plates can protect against it. Gamma radiation is therefore electromagnetic, but again not comparable to what we are used to.
So radioactivity means that one element becomes at least two others. The process can take a long time, in some cases since the formation of the earth. It can also be caused artificially, e.g. by bombarding it with elementary
particles. However, only neutrons are suitable for this, because protons and electrons would be deflected by their charge. You can also bombard smaller atomic nuclei than those with at least 84 protons. If new elements are
created by merging, the process is also called nuclear fusion.
An example of decay and fusion is the hydrogen bomb. The are ignited by an atomic bomb, in which conventional explosives are used to ignite material with an extremely heavy atomic nucleus, e.g. plutonium, is blown up to
a critical mass in order to then supply enough energy for the much more energetic nuclear fusion. The principle of the atomic bomb can be replicated in the appropriate power plants in a controlled manner, but nuclear
fusion, which is probably much less dangerous, cannot yet.
The unit becquerel, which is based on the number of decaying atomic nuclei per second, is known from accidents with nuclear power plants, e.g. in Fukushima. Then there's half-life, which also exists in fields other than
nuclear physics. Incidentally, the time in which only half of a nucleus is left usually decreases exponentially.
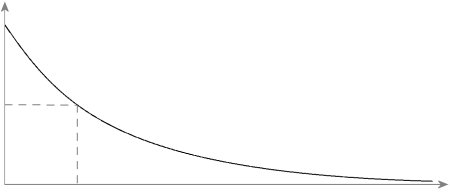
Here in the diagram, the number of particles still present before decay is put on the y-axis and the decay time is put on the x-axis. At the dashed line, only half is left, giving the half-life below. For the sake of completeness, it
should also be mentioned that there are also β and γ rays, the latter of which is probably by far the most difficult to shield.
| Isotopes | Atoms whose atomic number is the same and mass number is different, i.e. they differ only in the number of neutrons. |
| Mass number | The sum of the protons and neutrons in the nucleus corresponds to the atomic mass, with that of the other particles and electrons being negligibly small. |
| Nuclides | The sum of the protons and neutrons of the nucleus corresponds to the mass number. |
As you can see from the first entry in the box above, not only the ratio of protons to neutrons is different, there is also one and the same element with a different number of neutrons in the atomic nucleus. Only the isotopes of
hydrogen have their own names, such as 'deuterium' and 'tritium', which means nothing other than 'the second' and 'the third', i.e. hydrogen with one or two neutrons.
For all other isotopes, the percentage increase in mass by the addition of a neutron is not as great as that of hydrogen. The number of isotopes can also be significantly larger here. A uranium atom can have up to 24
different numbers of neutrons. The resulting isotopes have half-lives between one microsecond and one hour.
|
|