|
|
Inert Gases
1
H | | 2
He |
3
Li | 4
Be | | 5
B | 6
C | 7
N | 8
O | 9
F | 10
Ne |
11
Na | 12
Mg | | 13
Al | 14
Si | 15
P | 16
S | 17
Cl | 18
Ar |
19
K | 20
Ca | 21
Sc | 22
Ti
| 23
V | 24
Cr | 25
Mn | 26
Fe | 27
Co | 28
Ni | 29
Cu | 30
Zn |
31
Ga | 32
Ge | 33
As | 34
Se | 35
Br
| 36
Kr |
37
Rb | 38
Sr | 39
Y | 40
Zr | 41
Nb | 42
Mo | 43
Tc | 44
Ru | 45
Rh | 46
Pd | 47
Ag | 48
Cd | 49
In | 50
Sn | 51
Sb | 52
Te | 53
I | 54
Xe |
55
Cs | 56
Ba | 57-
71 | 72
Hf | 73
Ta | 74
W | 75
Rn | 76
Os | 77
Ir | 78
Pl | 79
Au | 80
Hg | 81
Tl | 82
Pb | 83
Bi | 84
Po | 85
At |
86
Rn |
87
Fr | 88
Ra | 89-
103 | 104
Rf |
105
Db | 106
Sg | Bh
108 | 108
Hs | 109
Mt | 110
Ds | 111
Rg | 112
Cn |
113
Uut | 114
Fl | 115
Uup | 116
Lv
| 117
Uus | 118
Uuo |
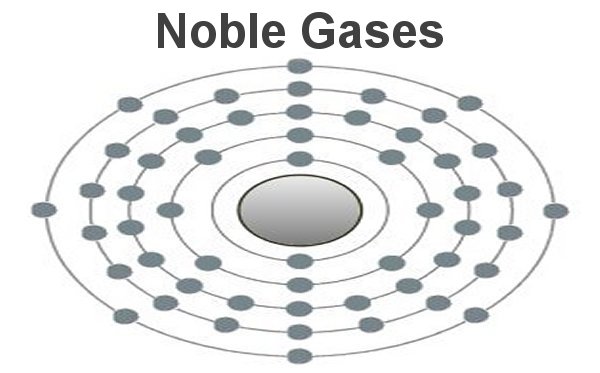
These are the inert gases. Their name is derived from the fact that they have the property, of not reacting with any other element. On the outside shells of their atoms all the places are occupied by electrons, a
special status in the periodic system. We can perceive inert gases then, when a current flows through them and they light up in the most wonderful colours. This occurs because the current-flow causes their
molecules to oscillate. Apart from Argon, they are relatively seldom.
Although it could be slotted in well at the end of the periodic system, the last natural occurring element was only discovered at the end of Mendelejew's creative period. This is the radioactive Radon. In the
automotive field, apart from being used for lighting purposes, inert gases are also used as shielding gases. In this case, e.g., as far as welding is concerned, they prevent other atmospheric gases from reaching
the weld. 03/13
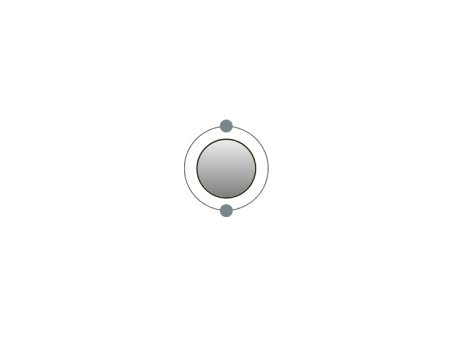
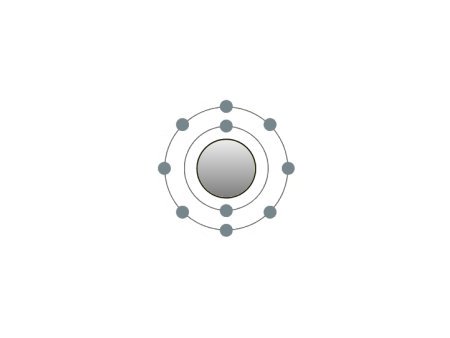
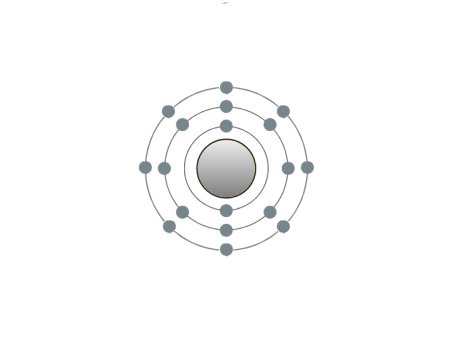
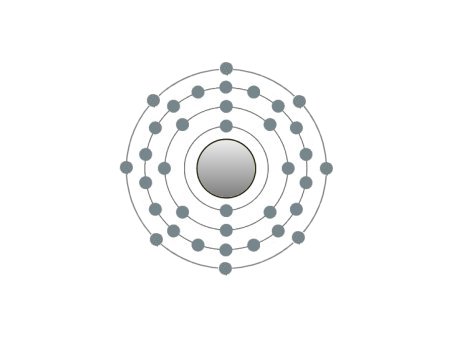
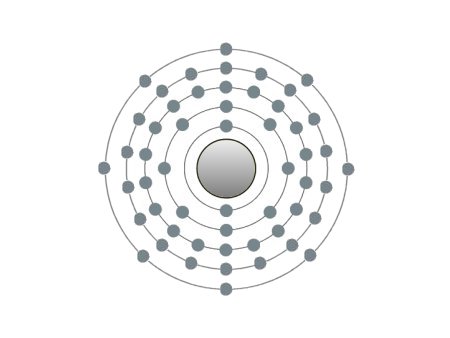
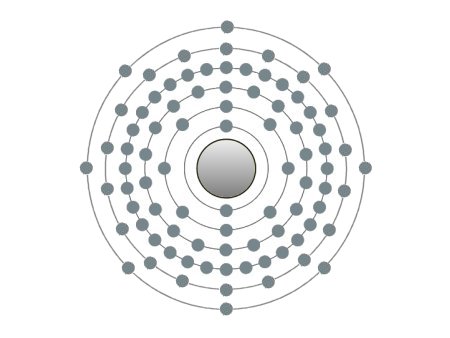
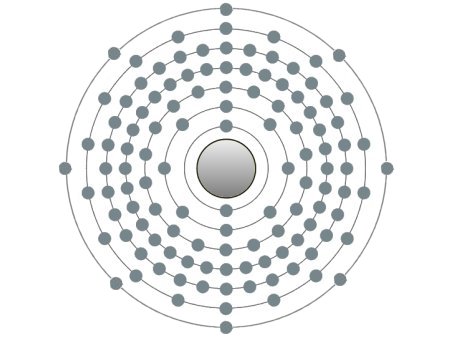
| If the atomic core was the size of a cherry, the entire atom would be as big as the Cologne Cathedral.
|
|
|